What Does An Acid Feel Like Chemistry . acids are substances that donate protons (h⁺ ions) or accept electron pairs. acids taste sour, e.g., citrus fruits taste source because of citrus acid and ascorbic acid, i.e., vitamin c, in them. Common examples include vinegar (acetic acid, ch₃cooh),. acid is another name for lysergic acid diethylamide (lsd), a hallucinogenic substance that temporarily alters a person’s mental state. Basic (alkaline) substances, on the other hand, taste bitter. acidic substances are usually identified by their sour taste. An acid is basically a molecule which can donate an h + ion and can remain energetically favourable after a loss of h. some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. In chemistry, acids and bases have been defined differently by three sets of theories. how does one define acids and bases?
from www.masterorganicchemistry.com
Basic (alkaline) substances, on the other hand, taste bitter. acids are substances that donate protons (h⁺ ions) or accept electron pairs. acid is another name for lysergic acid diethylamide (lsd), a hallucinogenic substance that temporarily alters a person’s mental state. how does one define acids and bases? In chemistry, acids and bases have been defined differently by three sets of theories. some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. Common examples include vinegar (acetic acid, ch₃cooh),. An acid is basically a molecule which can donate an h + ion and can remain energetically favourable after a loss of h. acidic substances are usually identified by their sour taste. acids taste sour, e.g., citrus fruits taste source because of citrus acid and ascorbic acid, i.e., vitamin c, in them.
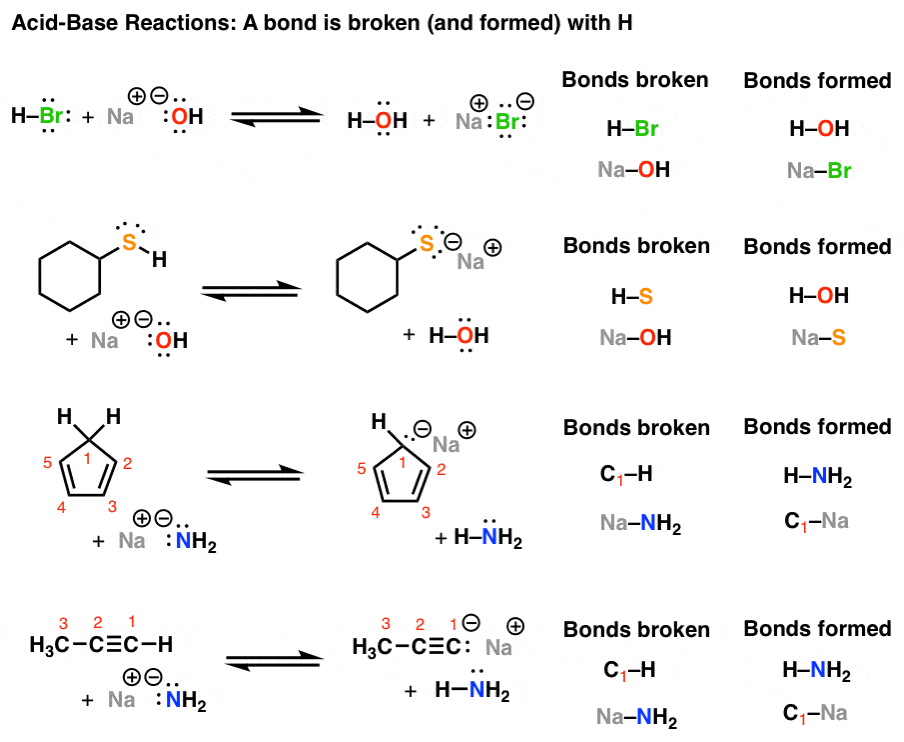
Introduction to AcidBase Reactions Master Organic Chemistry
What Does An Acid Feel Like Chemistry acids taste sour, e.g., citrus fruits taste source because of citrus acid and ascorbic acid, i.e., vitamin c, in them. acidic substances are usually identified by their sour taste. acids taste sour, e.g., citrus fruits taste source because of citrus acid and ascorbic acid, i.e., vitamin c, in them. In chemistry, acids and bases have been defined differently by three sets of theories. how does one define acids and bases? acid is another name for lysergic acid diethylamide (lsd), a hallucinogenic substance that temporarily alters a person’s mental state. acids are substances that donate protons (h⁺ ions) or accept electron pairs. Basic (alkaline) substances, on the other hand, taste bitter. Common examples include vinegar (acetic acid, ch₃cooh),. An acid is basically a molecule which can donate an h + ion and can remain energetically favourable after a loss of h. some acids are strong electrolytes because they ionize completely in water, yielding a great many ions.
From chem.libretexts.org
3.6b Naming Acids and Bases Chemistry LibreTexts What Does An Acid Feel Like Chemistry acids are substances that donate protons (h⁺ ions) or accept electron pairs. Basic (alkaline) substances, on the other hand, taste bitter. In chemistry, acids and bases have been defined differently by three sets of theories. how does one define acids and bases? Common examples include vinegar (acetic acid, ch₃cooh),. acidic substances are usually identified by their sour. What Does An Acid Feel Like Chemistry.
From www.teachoo.com
Uses of Acids 10+ Examples (with Images) Teachoo Teachoo Questio What Does An Acid Feel Like Chemistry An acid is basically a molecule which can donate an h + ion and can remain energetically favourable after a loss of h. acid is another name for lysergic acid diethylamide (lsd), a hallucinogenic substance that temporarily alters a person’s mental state. acids are substances that donate protons (h⁺ ions) or accept electron pairs. Common examples include vinegar. What Does An Acid Feel Like Chemistry.
From www.thoughtco.com
10 Common Acids and Chemical Structures What Does An Acid Feel Like Chemistry Basic (alkaline) substances, on the other hand, taste bitter. acids taste sour, e.g., citrus fruits taste source because of citrus acid and ascorbic acid, i.e., vitamin c, in them. An acid is basically a molecule which can donate an h + ion and can remain energetically favourable after a loss of h. some acids are strong electrolytes because. What Does An Acid Feel Like Chemistry.
From www.masterorganicchemistry.com
Introduction to AcidBase Reactions Master Organic Chemistry What Does An Acid Feel Like Chemistry Common examples include vinegar (acetic acid, ch₃cooh),. Basic (alkaline) substances, on the other hand, taste bitter. acids taste sour, e.g., citrus fruits taste source because of citrus acid and ascorbic acid, i.e., vitamin c, in them. acid is another name for lysergic acid diethylamide (lsd), a hallucinogenic substance that temporarily alters a person’s mental state. An acid is. What Does An Acid Feel Like Chemistry.
From www.reliableeducationgroups.in
Acids, Bases and Salts Class 7 Science Notes Chapter 5 Reliable What Does An Acid Feel Like Chemistry An acid is basically a molecule which can donate an h + ion and can remain energetically favourable after a loss of h. Common examples include vinegar (acetic acid, ch₃cooh),. acids are substances that donate protons (h⁺ ions) or accept electron pairs. Basic (alkaline) substances, on the other hand, taste bitter. some acids are strong electrolytes because they. What Does An Acid Feel Like Chemistry.
From pressbooks.bccampus.ca
6.3 AcidBase Reactions CHEM 1114 Introduction to Chemistry What Does An Acid Feel Like Chemistry Basic (alkaline) substances, on the other hand, taste bitter. Common examples include vinegar (acetic acid, ch₃cooh),. acids taste sour, e.g., citrus fruits taste source because of citrus acid and ascorbic acid, i.e., vitamin c, in them. In chemistry, acids and bases have been defined differently by three sets of theories. acids are substances that donate protons (h⁺ ions). What Does An Acid Feel Like Chemistry.
From www.sliderbase.com
The Chemistry of Acids and Bases Presentation Chemistry What Does An Acid Feel Like Chemistry how does one define acids and bases? acids are substances that donate protons (h⁺ ions) or accept electron pairs. some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. Common examples include vinegar (acetic acid, ch₃cooh),. An acid is basically a molecule which can donate an h + ion and can. What Does An Acid Feel Like Chemistry.
From www.thoughtco.com
List of Common Strong and Weak Acids What Does An Acid Feel Like Chemistry Common examples include vinegar (acetic acid, ch₃cooh),. how does one define acids and bases? In chemistry, acids and bases have been defined differently by three sets of theories. An acid is basically a molecule which can donate an h + ion and can remain energetically favourable after a loss of h. some acids are strong electrolytes because they. What Does An Acid Feel Like Chemistry.
From www.slideserve.com
PPT The Chemistry of Acids and Bases PowerPoint Presentation, free What Does An Acid Feel Like Chemistry acids taste sour, e.g., citrus fruits taste source because of citrus acid and ascorbic acid, i.e., vitamin c, in them. acidic substances are usually identified by their sour taste. An acid is basically a molecule which can donate an h + ion and can remain energetically favourable after a loss of h. In chemistry, acids and bases have. What Does An Acid Feel Like Chemistry.
From blog.praxilabs.com
Learn All About The Strong Acids and Bases PraxiLabs What Does An Acid Feel Like Chemistry Basic (alkaline) substances, on the other hand, taste bitter. An acid is basically a molecule which can donate an h + ion and can remain energetically favourable after a loss of h. acids are substances that donate protons (h⁺ ions) or accept electron pairs. acid is another name for lysergic acid diethylamide (lsd), a hallucinogenic substance that temporarily. What Does An Acid Feel Like Chemistry.
From www.online-sciences.com
Classification of Acids according to its strength (degree of ionization What Does An Acid Feel Like Chemistry An acid is basically a molecule which can donate an h + ion and can remain energetically favourable after a loss of h. acidic substances are usually identified by their sour taste. acid is another name for lysergic acid diethylamide (lsd), a hallucinogenic substance that temporarily alters a person’s mental state. how does one define acids and. What Does An Acid Feel Like Chemistry.
From study.com
Acidic, Basic & Neutral Solutions Determining pH Video & Lesson What Does An Acid Feel Like Chemistry acids taste sour, e.g., citrus fruits taste source because of citrus acid and ascorbic acid, i.e., vitamin c, in them. acidic substances are usually identified by their sour taste. some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. acid is another name for lysergic acid diethylamide (lsd), a hallucinogenic. What Does An Acid Feel Like Chemistry.
From studylib.net
AcidBase Chemistry What Does An Acid Feel Like Chemistry An acid is basically a molecule which can donate an h + ion and can remain energetically favourable after a loss of h. In chemistry, acids and bases have been defined differently by three sets of theories. acids are substances that donate protons (h⁺ ions) or accept electron pairs. acidic substances are usually identified by their sour taste.. What Does An Acid Feel Like Chemistry.
From studyschoolcasimere.z14.web.core.windows.net
Naming And Writing Formulas For Acids What Does An Acid Feel Like Chemistry An acid is basically a molecule which can donate an h + ion and can remain energetically favourable after a loss of h. acids taste sour, e.g., citrus fruits taste source because of citrus acid and ascorbic acid, i.e., vitamin c, in them. Basic (alkaline) substances, on the other hand, taste bitter. Common examples include vinegar (acetic acid, ch₃cooh),.. What Does An Acid Feel Like Chemistry.
From alevelchemistry.co.uk
Acids Facts, Summary, Weak & Strong ALevel Chemistry Revision What Does An Acid Feel Like Chemistry how does one define acids and bases? acid is another name for lysergic acid diethylamide (lsd), a hallucinogenic substance that temporarily alters a person’s mental state. some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. acids taste sour, e.g., citrus fruits taste source because of citrus acid and ascorbic. What Does An Acid Feel Like Chemistry.
From studylib.net
Acids and Bases What Does An Acid Feel Like Chemistry Basic (alkaline) substances, on the other hand, taste bitter. acidic substances are usually identified by their sour taste. some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. In chemistry, acids and bases have been defined differently by three sets of theories. how does one define acids and bases? acids. What Does An Acid Feel Like Chemistry.
From leah4sci.com
Acids and Bases in Organic Chemistry What Does An Acid Feel Like Chemistry Basic (alkaline) substances, on the other hand, taste bitter. An acid is basically a molecule which can donate an h + ion and can remain energetically favourable after a loss of h. acid is another name for lysergic acid diethylamide (lsd), a hallucinogenic substance that temporarily alters a person’s mental state. Common examples include vinegar (acetic acid, ch₃cooh),. . What Does An Acid Feel Like Chemistry.
From www.chemistrylearner.com
AcidBase Reaction Definition, Examples, and Uses What Does An Acid Feel Like Chemistry Basic (alkaline) substances, on the other hand, taste bitter. Common examples include vinegar (acetic acid, ch₃cooh),. acidic substances are usually identified by their sour taste. some acids are strong electrolytes because they ionize completely in water, yielding a great many ions. acid is another name for lysergic acid diethylamide (lsd), a hallucinogenic substance that temporarily alters a. What Does An Acid Feel Like Chemistry.